Ozone Layer Protection and You
What is meant by "ozone hole" ?
Since about 1975, scientists have detected a severe drop in ozone concentration in the layer over the Antarctica each spring. The situation then reached an alarming scale in 1987 when an international expedition found that half of the Antarctica's ozone have disappeared over a region twice the size of the United States, creating an enormous "hole" in the ozone layer. Concentrations of ozone fell by as much as 50% of the norm at altitude of 18 km. At mid-latitudes in the Northern Hemisphere, up to 3% decrease in ozone concentration was also observed.
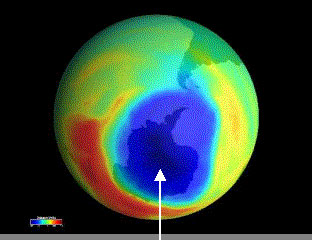 |
| (Severe Drop in Ozone Concentration) |
Why it concerns you?
The ozone molecules form a protective layer which extends from about 16 km to 50 km up above the earth at low latitudes, and from about 8 km to 50 km at high latitudes. The ozone molecules absorb the sun's ultra violet radiation (UV) which will be harmful to us if it reaches the earth surface. With more UV radiation reaching the earth surface due to ozone depletion, human health and the environment will be adversely affected. The most significant effects will be the increased incidence of skin cancer, eye cataracts, damage to the human immune system and to the ecology of the earth.
What causes this phenomenon?
Scientists have reached consensus that ozone depletion in the stratosphere is caused by ozone depleting chemicals. These chemicals contain chlorine or bromine atom with inherent chemical stability and have long lifetime in the atmosphere, in the range of 40 to 150 years. These chemicals and other trace gases drift up into the stratosphere and become involved in chlorine-releasing reactions. The chlorine atoms then react with the ozone molecules in the presence of sunlight and destroy the ozone molecules. Just one chlorofluorocarbon molecule can destroy tens of thousands of ozone molecules.
These ozone-depleting chemicals are extensively used man-made chemicals including the followings: -
-
chlorofluorocarbons (CFCs);
-
halons;
-
1,1,1-trichloroethane (methyl chloroform);
-
carbon tetrachloride;
-
methyl bromide;
-
hydrobromofluorocarbons (HBFCs); and
-
hydrochlorofluorocarbons (HCFCs).
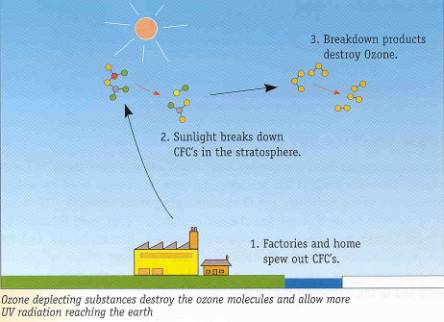
What are these ozone depleting substances (ODS) used for?
The following are the common usage of CFCs and HCFCs :
- CFC-11, CFC-12 and HCFC-22 are used as cooling agents in home refrigerators, retail store refrigeration systems, chillers and air-conditioners.
- CFC-11 and CFC-12 are used as propellants for aerosol sprays such as hair mousses and household cleaning products.
- CFC-11 and CFC-12 are also used as blowing agents in the manufacture of foams for home furnishing, insulation and packaging. Some plastics may be shaped using CFCs, e.g. egg cartons, cups and cartons used in fast food operations. Rigid or semi-rigid foams are also used as thermal or sound insulation in refrigeration equipment, buildings and automobiles.
- CFC-113 is a solvent for cleaning electronic circuit boards and computer components.
- solvent for cleaning electronic circuit boards and metal work such as watches and clockworks.
- thinner such as that for correction fluid.
- cleaning agent in the textile industry (dry cleaning).
Carbon tetrachloride is used as a cleaning agent in textile and electronics industries.
Can we get rid of the ODS?
There has been considerable progress in finding non-ozone-depleting substitutes for ODS in the last few years. Substitutes for air-conditioning and refrigeration applications are now available, such as that CFC-12 can be replaced by HFC-134a. There are also emerging markets for "drop-in" replacement for CFC and halons.
Alternative products or processes can be used in some cases including the following:
-
alternative insulating materials;
-
substitute food containers such as hydrocarbon blown polystyrene, plastic film wrap and bags;
-
alternative packaging materials such as plastic film bubble wraps; and
-
air-conditioning and refrigeration plants operating on non-CFC refrigerants.
CFC solvents can be substituted in some applications. For instance, petroleum solvents can be selected as a replacement for CFC-113 or 1,1,1-trichloroethane in cleaning applications. Aqueous cleaning, or even no-clean technology, are also alternative processes that can be used by the electronics industry. Many household and personal aerosol products, e.g. paint sprays and insecticides, now use hydrocarbons (e.g. propane and butane) as propellants instead of CFCs. Dimethyl ether may also be used as propellants replacing CFCs.
What are the international efforts in saving the ozone layer?
In September 1987, an international treaty aimed at saving the Earth's ozone layer, known as the Montreal Protocol on Substances that Deplete the Ozone Layer, was signed in Montreal, Canada. The Protocol requires the phasing out of the ODS in accordance with agreed schedules. Following lists the ODS phasing out schedules applicable to Hong Kong:
|
Halons |
Import for local consumption banned by 1.1.1994 |
|
CFCs Carbon Tetrachloride 1,1,1-Trichloroethane |
Import for local consumption banned by 1.1.1996 |
|
HCFCs |
Freeze consumption at base level starting 1.1.1996 Import for local consumption banned by 1.1.2030 |
How does Hong Kong control the ODS?
To fulfil Hong Kong Special Administrative Region's international obligations under the 1985 Vienna Convention for the Protection of the Ozone Layer and the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer, the Ozone Layer Protection Ordinance was enacted in July 1989 to provide a statutory framework for the control of ozone depleting substances. The chemicals under control are referred to as "scheduled substances" in the Ordinance (Please see Appendix 1). The Ordinance prohibits the manufacturing of such substances and imposes controls on the import and export of these substances through registration and licensing provisions. The following is a summary of the related control:
| Measures | Commencement Date |
|---|---|
| Control of import and export of scheduled substances |
1.7.1989 |
| Banning of import for local consumption of halons |
1.1.1994 |
| Licensing of import of methyl bromide strictly for local quarantine and pre-shipment applications |
1.1.1995 |
| Banning of import for local consumption of CFCs, 1,1,1-trichloroethane,carbon tetrachloride and HBFCs |
1.1.1996 |
| Licensing of import of HCFCs for local consumption |
1.1.1996 |
Under the registration and licensing system, persons who wish to import or export any of the ODS must:
-
first register with the Trade and Industry Department,
-
apply for a licence from the Trade and Industry Department on each occasion, and/or
-
possess the quota to import the HCFC for local consumption .
In 1993, two pieces of legislation were introduced under the Ozone Layer Protection Ordinance:
-
The Ozone Layer Protection (Products Containing Scheduled Substances) (Import Banning) Regulation
-
The Ozone Layer Protection (Controlled Refrigerants) Regulation
Copies of the Ozone Layer Protection Ordinance and subsidiary regulations are on sale at the Government Publications Centre. Also, they can be browsed from the web site of Bilingual Laws Information System at http://www.legislation.gov.hk.
What is the Ozone Layer Protection (Products Containing Scheduled Substances) (Import Banning) Regulation about?
This Regulation prohibits the import of products containing CFCs and halons from countries which are not Parties to the Montreal Protocol:
-
an air-conditioner or heat pump designed to cool the driver's or passengers' compartment of a motor vehicle (whether or not installed in the motor vehicle);
-
refrigeration equipment or air-conditioning or heat pump equipment (whether for domestic or commercial use);
-
an aerosol product other than an aerosol product containing a pharmaceutical product or medicine as defined in section 2 of the Pharmacy and Poisons Ordinance (Cap. 138);
-
insulation panel, insulation board or insulation pipe cover;
-
a pre-polymer;
-
portable fire extinguishers containing halon FROM ANY COUNTRIES.
What is the Ozone Layer Protection (Controlled Refrigerants) Regulation about?
This Regulation prohibits any intended release of controlled refrigerants from motor vehicle air-conditioners or refrigeration equipment containing more than 50 kg of refrigerant charge into the atmosphere, and to conserve the controlled refrigerants through the use of approved recycling and recovery equipment.
For enforcement and monitoring purposes, owners or operators of industrial/commercial refrigeration systems, as well as proprietors of garages, shall be required to keep records on relevant repair services and the amount of CFC-based refrigerants consumed. Proprietors of vehicle scrap-yards shall also be required to keep records on the number of motor vehicle air conditioners decommissioned as well as the amount of CFC-based refrigerants recovered from the decommissioned air conditioners.
How can I help to protect the ozone layer?
While the vast majority of ODS usage is either industrial or commercial, individuals can help in the following ways:
- Buy air-conditioning equipment that does not use CFC as refrigerant.
-
Conduct regular inspection and maintenance of air-conditioning and refrigeration appliances to prevent and minimize refrigerant leakage.
- For existing air-conditioning and refrigeration appliances that operate on CFCs, the refrigerant should be recovered or recycled whenever an overhaul of equipment is to be carried out. Replacing or retrofitting such equipment to operate on non-CFC refrigerant should also be considered.
- When motor vehicle air-conditioners need servicing, make sure that the CFC refrigerants are properly recovered and recycled instead of being vented to the atmosphere.
- When using CFC-113 as solvent or cleaning agent, make sure that the chemical is properly handled so that evaporation losses are minimized and the waste solution is recycled as far as possible. Alternative technologies such as aqueous cleaning should also be considered.
FURTHER INFORMATION
Enquiries concerning the Ozone Layer Protection Ordinance and any other general information on the registration and licensing provisions may be made to the Air Policy Group of Environmental Protection Department at the following address:
| Address | Telephone | Facsimile |
|---|---|---|
| 33/F, Revenue Tower, | 2594 6261 | 2827 8040 |
| 5 Gloucester Road, | 2594 6218 | |
| Wan Chai, Hong Kong | 2594 6275 |
Enquiries regarding the application for registration and import or export licences may be made to the Non-textiles Licensing Unit of Trade and Industry Department at the following address:
| Address | Telephone | Facsimile |
|---|---|---|
| Room 101A, 1/F., Trade and Industry Department Tower, 700 Nathan Road, Kowloon. |
2398 5560
|
2380 8504 |
December, 2005
Acknowledgement:
Permission to use the image of "ozone hole" from the Ozone Processing Team, Goddard Space Flight Centre, NASA, and the ozone depleting illustration schematic from the Centre for Atmospheric Science, Chemistry Dept., University of Cambridge ("Ozone Hole Tour" website address, http://www.atm.ch.cam.ac.uk/tour/) is gratefully acknowledged.
Appendix 1
SCHEDULE
Scheduled Substances
PART 1
Chlorofluorocarbons (CFC)
| Chemical Name | Common Name | |
|---|---|---|
|
CFCl3 |
Trichlorofluoromethane |
CFC-11 |
|
CF2Cl2 |
Dichlorodifluoromethane |
CFC-12 |
|
C2F3Cl3 |
Trichlorotrifluoroethane |
CFC-113 |
|
C2F4Cl2 |
Dichlorotetrafluoroethane |
CFC-114 |
|
C2F5Cl |
Chloropentafluoroethane |
CFC-115 |
PART 2
Halons
| Chemical Name | Common Name | |
|---|---|---|
|
CF2BrCl |
Bromochlordifluoromethane |
halon 1211 |
|
CF3Br |
Bromotrifluoromethane |
halon 1301 |
|
C2F4Br2 |
Dibromotetrafluoroethane |
halon 2402 |
PART 3
Other Fully Halogenated Chlorofluorocarbons
| Chemical Name | Common Name | |
|---|---|---|
|
CF3Cl |
Chlorotrifluoromethane |
CFC-13 |
|
C2FCl5 |
Pentachlorofluoroethane |
CFC-111 |
|
C2F2Cl4 |
Tetrachlorodifluoroethane |
CFC-112 |
|
C3FCl7 |
Heptachlorofluoropropane |
CFC-211 |
|
C3F2Cl6 |
Hexachlorodifluoropropane |
CFC-212 |
|
C3F3Cl5 |
Pentachlorotrifluoropropane |
CFC-213 |
|
C3F4Cl4 |
Tetrachlorotetrafluoropropane |
CFC-214 |
|
C3F5Cl3 |
Trichloropentafluoropropane |
CFC-215 |
|
C3F6Cl2 |
Dichlorohexafluoropropane |
CFC-216 |
|
C3F7Cl |
Chloroheptafluoropropane |
CFC-217 |
PART 4
Methyl Chloroform
| Chemical Name | Common Name | |
|---|---|---|
|
C2H3Cl3 |
1,1,1-Trichloroethane |
Methyl Chloroform |
PART 5
Carbon Tetrachloride
| Chemical Name | Common Name | |
|---|---|---|
|
CCl4 |
Tetrachloromethane |
Carbon Tetrachloride |
PART 6
Methyl Bromide
| Chemical Name | Common Name | |
|---|---|---|
|
CH3Br |
Bromomethane |
Methyl Bromide |
PART 7
Hydrobromofluorocarbons (HBFC)
| Chemical Name | Common Name | |
|---|---|---|
|
CHFBr2 |
||
|
CHF2Br |
Bromodifluoromethane |
HBFC-22B1 |
|
CH2FBr |
Bromofluoromethane |
--- |
|
C2HFBr4 |
Tetrabromofluoroethane |
--- |
|
C2HF2Br3 |
Tribromodifluoroethane |
--- |
|
C2HF3Br2 |
Dibromotrifluoroethane |
--- |
|
C2HF4Br |
Bromotetrafluoroethane |
--- |
|
C2H2FBr3 |
Tribromofluoroethane |
--- |
|
C2H2F2Br2 |
Dibromodifluoroethane |
--- |
|
C2H2F3Br |
Bromotrifluoroethane |
--- |
|
C2H3FBr2 |
Dibromofluoroethane |
--- |
|
C2H3F2Br |
Bromodifluoroethane |
--- |
|
C2H4FBr |
Bromofluoroethane |
--- |
|
C3HFBr6 |
Hexabromofluoropropane |
--- |
|
C3HF2Br5 |
Pentabromodifluoropropane |
--- |
|
C3HF3Br4 |
Tetrabromotrifluoropropane |
--- |
|
C3HF4Br3 |
Tribromotetrafluoropropane |
--- |
|
C3HF5Br2 |
Dibromopentafluoropropane |
--- |
|
C3HF6Br |
Bromohexafluoropropane |
--- |
|
C3H2FBr5 |
Pentabromofluoropropane |
--- |
|
C3H2F2Br4 |
Tetrabromodifluoropropane |
--- |
|
C3H2F3Br3 |
Tribromotrifluoropropane |
--- |
|
C3H2F4Br2 |
Dibromotetrafluoropropane |
--- |
|
C3H2F5Br |
Bromopentafluoropropane |
--- |
|
C3H3FBr4 |
Tetrabromofluoropropane |
--- |
|
C3H3F2Br3 |
Tribromodifluoropropane |
--- |
|
C3H3F3Br2 |
Dibromotrifluoropropane |
--- |
|
C3H3F4Br |
Bromotetrafluoropropane |
--- |
|
C3H4FBr3 |
Tribromofluoropropane |
--- |
|
C3H4F2Br2 |
Dibromodifluoropropane |
--- |
|
C3H4F3Br |
Bromotrifluoropropane |
--- |
|
C3H5FBr2 |
Dibromofluoropropane |
--- |
|
C3H5F2Br |
Bromodifluoropropane |
--- |
|
C3H6FBr |
Bromofluoropropane |
--- |
PART 8
Hydrochlorofluorocarbons (HCFC)
| Chemical Name | Common Name | |
|---|---|---|
|
CHFCl2 |
Dichlorofluoromethane |
HCFC-21 |
|
CHF2Cl |
Chlorodifluoromethane |
HCFC-22 |
|
CH2FCl |
Chlorofluoromethane |
HCFC-31 |
|
C2HFCl4 |
Tetrachlorofluoroethane |
HCFC-121 |
|
C2HF2Cl3 |
Trichlorodifluoroethane |
HCFC-122 |
|
C2HF3Cl2 |
Dichlorotrifluoroethane |
HCFC-123 |
|
C2HF4Cl |
Chlorotetrafluoroethane |
HCFC-124 |
|
C2H2FCl3 |
Trichlorofluoroethane |
HCFC-131 |
|
C2H2F2Cl2 |
Dichlorodifluoroethane |
HCFC-132 |
|
C2H2F3Cl |
Chlorotrifluoroethane |
HCFC-133 |
|
C2H3FCl2 |
Dichlorofluoroethane |
HCFC-141 |
|
C2H3F2Cl |
Chlorodifluoroethane |
HCFC-142 |
|
C2H4FCl |
Chlorofluoroethane |
HCFC-151 |
|
C3HFCl6 |
Hexachlorofluoropropane |
HCFC-221 |
|
C3HF2Cl5 |
Pentachlorodifluoropropane |
HCFC-222 |
|
C3HF3Cl4 |
Tetrachlorotrifluoropropane |
HCFC-223 |
|
C3HF4Cl3 |
Trichlorotetrafluoropropane |
HCFC-224 |
|
C3HF5Cl2 |
Dichloropentafluoropropane |
HCFC-225 |
|
C3HF6Cl |
Chlorohexafluoropropane |
HCFC-226 |
|
C3H2FCl5 |
Pentachlorofluoropropane |
HCFC-231 |
|
C3H2F2Cl4 |
Tetrachlorodifluoropropane |
HCFC-232 |
|
C3H2F3Cl3 |
Trichlorotrifluoropropane |
HCFC-233 |
|
C3H2F4Cl2 |
Dichlorotetrafluoropropane |
HCFC-234 |
|
C3H2F5Cl |
Chloropentafluoropropane |
HCFC-235 |
|
C3H3FCl4 |
Tetrachlorofluoropropane |
HCFC-241 |
|
C3H3F2Cl3 |
Trichlorodifluoropropane |
HCFC-242 |
|
C3H3F3Cl2 |
Dichlorotrifluoropropane |
HCFC-243 |
|
C3H3F4Cl |
Chlorotetrafluoropropane |
HCFC-244 |
|
C3H4FCl3 |
Trichlorofluoropropane |
HCFC-251 |
|
C3H4F2Cl2 |
Dichlorodifluoropropane |
HCFC-252 |
|
C3H4F3Cl |
Chlorotrifluoropropane |
HCFC-253 |
|
C3H5FCl2 |
Dichlorofluoropropane |
HCFC-261 |
|
C3H5F2Cl |
Chlorodifluoropropane |
HCFC-262 |
|
C3H6FCl |
Chlorofluoropropane |
HCFC-271 |