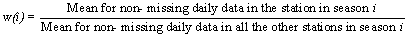Environmental Health and
Biostatistics and Computing Groups
Department of Community Medicine
The University of Hong Kong
Project Team
| Dr CM Wong (Data analysis and report writing) |
Mr Stefan Ma (Computation and statistical advice) |
| Professor AJ Hedley (Head of Department) |
Professor TH Lam (Epidemiological advice) |
CONTENTS
Executive Summary
References
Figures
Basic Tables
Operation Manual
Executive Summary
Background and objectives
Valuable indicators of the possible benefits of environmental management and control can be obtained by extrapolation from analysis carried out in other locations; but governments and local regulatory agencies are usually unable to draft or implement effective legislation without relevant local information to support their proposals. A study had been commissioned to the Chinese University of Hong Kong (CUHK) by the Environmental Protection Department, to evaluate the acute health effects of air pollution, using data for 1994-96 as a first attempt towards utilizing local intelligence. This study is a follow-up of the first study, aiming to validate the methods and results in the first study.
Methods
A series of daily hospital admissions and deaths in 1995, 1996 and the first half of 1997, due to respiratory and circulatory diseases, were obtained from routinely collected data and were analysed using Poisson regression with adjustment for overdispersion and long term effects of covariates
(including trend, seasonality, weekdays, holidays, after holidays, temperature and humidity). The health effects due to daily pollutant concentrations of sulphur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3) and respirable suspended particulates (RSP) were then estimated and compared with those obtained from other similar studies.
Findings
| (a) | For hospital admissions Air pollution was found to have an effect on circulatory and respiratory diseases combined and separately (relative risk, RR=1.03-1.10; p<0.084) for all ages; on circulatory admissions (RR=1.05-1.10; p<0.001) for the 65 or above age group; on respiratory admissions, the effects of which appeared to be j-shaped from the younger to the older age groups; on asthma (RR=1.10-1.16; p<0.018, except SO2); on chronic obstructive pulmonary disease (RR=1.08-1.14; p<0.0001); and on ischaemic heart disease (RR=1.04-1.09; p<0.051, except SO2 and RSP). |
| (b) | For hospital deaths Both NO2 and O3 were positively associated with circulatory and respiratory diseases combined and separately (for NO2: RR= 1.10-1.14, p<0.038; and for O3: RR=1.07-1.22, p<0.010). |
| (c) | Validation and composite score The above estimates were consistent with and in between those obtained from similar studies using the European (APHEA) approach overseas and in the CUHK. But in addition, a composite score was derived from the four pollutants and was found to provide consistent estimates for all the health outcomes under study in all ages (RR=1.04-1.11, p<0.098, except hospital deaths due to circulatory diseases). |
Conclusions
Routine hospital morbidity and mortality data, air pollution and meteorological observations can be utilized to provide information for the estimation of acute health effects of air pollution. Environmental management and control should and could take into account health effects of air pollution based on locally derived information. However the processes are errors prompted (as large and complex data sets are involved) and are vulnerable to the misuse of health and other parameters and to misinterpretation of the results as it involves knowledge from several different fields. A team approach with expertise from epidemiological, environmental, statistical and computational professionals is required.
1.0 Background and introduction
| 1.1 | The contribution of epidemiological studies to the process of environmental management and control of health hazards is well established world-wide. Valuable indicators of possible benefits can be obtained by extrapolation from analyses carried out in other locations but, in general, governments and local regulatory agencies are unable to draft or implement effective legislation without relevant local information to support their proposals. |
| 1.2 | Several epidemiological studies have now shown an association between particulate air pollution and exacerbations of illness in individuals with respiratory disease and also increases in the numbers of deaths from cardiovascular and respiratory disease, particularly in the elderly. New hypotheses have been advanced to postulate mechanisms underlying these observed effects (Seaton et al 1995).1 Respirable particulates (RSP) with an aerodynamic diameter of <10 um(or particles measured as black smoke by the smoke stain method) comprise the principal pollutant associated with these findings. In addition RSP sulphate and sulphur dioxide concentrations, are reported to be associated with all causes mortality and respiratory mortality in recent studies from the USA (Pope et al 1995)2 and the UK (Anderson et al 1996)3 respectively. A recent review in the United Kingdom concludes that the associations between daily concentrations of particles and acute health effects principally reflect a causal relationship (Committee on the Medical Effects of Air Pollutants 1995).4 After a lengthy scientific review, the USEPA determined that new standard should be added for particulates less than 2.5 ? of aerodynamic in size and the welfare-base standards were also revised by making them identical to the health-based standards.5 In Hong Kong we have found that SO2, RSP and SO4 concentrations are associated with excess risks for symptoms of cough, phlegm and wheeze and also bronchial hyper-responsiveness (by histamine challenge test) in primary school children (Hedley et al 1993; Peters et al 1996; Tam et al 1994; Wong et al 1998).6,7,8,9 However in the London study the strongest association with daily mortality was for ozone. The effects of ozone and black smoke were independent of the effects of other pollutants. Evidence on the risks associated with other pollutants is variable and less consistent. In the recent London study (Anderson et al 1996)3 the NO2 (1 hour maximum) was associated with all causes mortality and cardiovascular mortality; however a negative effect was seen for respiratory mortality. A significant positive effect on mortality was seen for SO2 and all cause mortality in the warm season period. Several authors point to the complex between-season covariation of several pollutants, which is sometimes negative and at other times positive. |
| 1.3 | The published literature in this field is growing rapidly. The Department of Community Medicine is monitoring this through different databases and will aim to carry out the analysis using a state-of-the-art approach. This will enhance the utility of the outputs and ensure comparability as far as possible with studies in other countries (Katsouyanni, Schwartz, Spix et al 1996).10 |
2.0 Scope and objectives
| 2.1 | to examine the variation of daily air pollution data i.e. 24 hour average for sulphur dioxide, nitrogen dioxide and respirable suspended particulates and 8 hour average for ozone, among the various monitoring stations in Hong Kong for the years 1995-1996, as available; |
| 2.2 | to investigate the availability and the use of the various health outcome measures including data on hospital admissions and hospital deaths due to respiratory and circulatory problems collected routinely in the Hong Kong hospitals; |
| 2.3 | to investigate the short-term effects of the air pollutants considered in 2.1 (in the same day and one or more days lagged) individually and compositely on some of the health outcome measures considered in 2.2 above, with adjustment for seasonal variations, secular trends as well as meteorological conditions including temperature and humidity; |
| 2.4 | to validate and update models developed earlier and to develop a mechanism for the use and maintenance of the model for continuous study by the Environmental Protection Department. |
3.0 Materials and methods
| 3.1 | Study design It was an ecological study utilizing routinely collected hospital admission data, air pollutant concentration data and weather data by the Hospital Authority, Environmental Protection Department and the Observatory respectively. Variations in the daily number of hospital admissions due to circulatory and respiratory diseases were studied, and their relationships with each of the pollutants were modelled to assess the effects of air pollution on health after adjustment for time trends, seasonality, weather conditions and some other factors including days of week, holidays and days after holidays. This study follows a previous one performed by the Chinese University of Hong Kong (CUHK)11 which followed the general approach of the protocol of the APHEA (a European approach using epidemiological time series data), developed within the frame of the EC Environment 1991-94 Programme. However data for some disease categories (ICD Rubrics) included in the APHEA protocol were not analysed in the CUHK study. The data sets and disease categories used for this new study are shown in Table 1 below. Those categories which were excluded or missing from the CUHK study are indicated in the table.
** data for ICD9 390-392, 393-398, 446-448, 451-459, 470 and 510-519 were not included for analysis in the CUHK Final Report *** data for ICD9 401-405 and 500-508 were missing in the CUHK data files and were not analysed in the CUHK Final Report ( ) CUHK data in brackets - not included for analysis in the CUHK study In order not to exclude categories which might show effects from air pollution (e.g. hospital admissions for cardiovascular diseases under ICD9 390 - 429 which were found to be related to respirable suspended particulates and carbon monoxide by Schwartz (1997)13 but not all included in the CUHK study) and to ensure comparability to the results of those studies which follow the APHEA protocol (Bacharova 1996; Schouten 1996),14,15 we decided to follow strictly the categories recommend by the APHEA protocol for this study. Data for 1995-1996 (generated and cleaned by the Department of Community Medicine, the University of Hong Kong in this study) were used in establishing the statistical models and in estimating the effects; and data for first half year of 1997 were used for validation of the established models. It was also decided that data for the year 1994, which were used by the CUHK group, should not be used in this study because the data quality is not consistent with that of the 1995-96 data set to be used in this study. In 1994 only 3 of the 12 hospitals under study had already adopted the MRAS database; but the number increased to 7 in 1995. Besides, the percentages of valid daily data for air pollutant concentrations from the monitoring stations were lower in 1994 than those in the other years. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 | Databases Hospital admission data: Hospitals included for generation of hospital admissions were the publicly funded hospitals (accounting for 90% of hospital beds in Hong Kong) which either had an accident and emergency department, or was a referral base from the accident and emergency department of another nearby hospital (9) or had a 24-hour outpatient department (2). One other hospital which was the only hospital in the most polluted district in Hong Kong, was also included. All hospitals should have a computerized system for inputting and retrieval of patient data. The hospitals included in the study, together with the type of information system they were using and an indication as to whether they had an A & E department, were listed in Table 2 below:
MRAS - Medical Records Abstracting System Patients admitted to hospitals between 1.1.1995 and 30.6.1997 with data on: dates of admission and discharge, socio-demographic information (age, gender, marital status, ethnic group, district of residence, pseudo identifier of patient), admission source, discharge diagnosis in ICD9 codes and discharge status were retrieved from the databases for each of the hospitals under study. In order to validate the completeness of the retrieved data, the total number of inpatients for the period 1.4.1995 to 31.3.1996, for each hospital, were compared with those reported in the Hospital Authority Statistical Report 1995/96. When there were big differences between the two, the Hospital Authority Information Technology Department was ask for an explanation and we then revised the databases if necessary until they were reasonably close to each other. This process took almost a half year to complete. The data are shown in Table 3 below:
@ The excess 5409 cases in the HKU data set were day cases which could not be excluded when the Information Technology Department generated the data set due to missing of the identifier. The total data sets were then extracted for circulatory (ICD9 390-459) and respiratory (ICD9 460-519) diseases. The numbers of hospital admissions by disease groups in the three years were as shown in the previous Table 1 and subset of specific disease categories are shown in Table 4 below:
Acute myocardial infarction was not analysed, as had been done by the CUHK, because diagnosis for the disease has been changing and subject to misclassification over the past years. Pollutant concentration data: Pollutant concentration data in CD-ROM were made available by the Air Services Group of the Environmental Protection Department with hourly data from all monitoring stations in Hong Kong. The following stations in various urban, suburban and industrial areas were included in the study:
In order to maintain consistency and similar quality standards in the data, missing data were defined and replaced in accordance with the APHEA recommendations. The guidelines were slightly modified to suit local situations and these are described in Table 7 below:
# A minimum of 67% non-missing daily data was used as criteria for inclusion of a pollutant in the analysis instead of 75%. This was set in the computer programme at the beginning of the study when the HKU was trying to adopt a similar procedure as that used by the CUHK. However this might not be necessary for data after 1994 as the data were more complete. The data in monthly averages were comparable to those in the EPD 1995 and 1996 statistical reports. The percentage of data valid after replacement are shown in the following Table 8:
- not available # Abbreviations referred to Table 5. A daily mean concentration representing the pollution level in all Hong Kong areas were obtained from valid data of all the stations for each of the pollutants under study. Meteorological data: Daily means for temperature and relative humidity were obtained from the Environmental Protection Department which derived the data from the Observatory. The monthly means for the years 1995 and 1996 were comparable with those reported in the Hong Kong Annual Reports. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 |
Statistical modellingThe statistical modelling methods followed the guidelines recommended by the APHEA protocol and are outlined as follows:
Back to Contents |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4.0 Findings
| 4.1 | Descriptive statistics Summary statistics of daily hospital admissions for circulatory and respiratory diseases, both combined and separately by 3 month periods are shown in Basic Table A5.1 to A5.3 (in Appendix). Summary statistics of all the health outcomes used in this study are shown in Tables 9-12 below:
COPD - Chronic obstructive pulmonary disease IHD - Ischaemic heart disease
# first half year COPD - Chronic obstructive pulmonary disease IHD - Ischaemic heart disease
# first half year Summary statistics of daily mean temperature, relative humidity, and mean concentration of each of the pollutants are shown in the Basic Tables B1-2 and C4-7. The correlations among the four pollutants, and between each pollutant and the health outcomes are shown in Table 13.
The correlations among the pollutant concentrations, temperature and humidity are shown in the following Table 14.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 | Statistical modelling Core models for hospital admissions: The following Tables 15-17 show the results of core models which explain the hospital admissions due to circulatory and respiratory disease categories in terms of daily linear and quadratic time trends, year effect, seasonality (using sine and cosine functions), day of week (compared with Sunday), holidays and days after the holidays, temperature and relative humidity. (Air pollutant concentrations are not included at this stage.) For the two disease groups, both combined and separately, there were positive daily linear and quadratic trends over the two year period; and weekdays (Monday to Friday relative to Sunday), day after holidays, higher temperature and lower humidity were positively associated with hospital admissions (p<0.012, except for Friday effects in respiratory admission where p=0.708). Each model explained 64-79% of the variation in the health outcomes. Similar effects were also found in individual age groups (younger than 15 years, 15-64 and 65 years and older age groups), for both disease categories separately, except in those younger than 15 years for respiratory diseases, (most p<0.10) (Basic Tables E 7-12). The above models explained 39-77% of the variations in the health outcomes. For admissions due to asthma, chronic obstructive pulmonary and ischaemic heart diseases the effects due to the whole set of meteorological, seasonality and trend were similar except that numbers of hospital admissions were associated with lower temperature (p<0.044) (Basic Tables E 4-6). The models explained 31% to 49% of the variations in the health outcome.
Models with pollutants for hospital admissions: The relative risks (RR) with 95% confidence intervals (95% CI) for hospital admissions in all ages due to each of the disease categories are shown in Table 18 below, with comparison to similar estimates from the CUHK reports. As the CUHK study used data of 1994-95 (instead of 1995-96) and included different ICD9 codes for circulatory and respiratory diseases, the results will not be directly comparable to those from the HKU study. All relative risks in the tests and in Tables 18-21 below were referred to 50 ug/m3 changes in concentration of the pollutants. However relative risks for 100 ug/m3 changes were also presented in Tables 18a-21a (Appendix B) for the pollutants correspondingly.
** p < 0.01; *** p < 0.001 # Notes: Lag0-n denotes the mean of cumulative effects of lag from day 0 (same day) up to previous n days using up to 5 days (Lag0-5) for ozone and up to 3 days (Lag0-3) for other pollutants The results will be discussed in 5.2-5.4. Models of pollutants and hospital deaths: The relative risks (RR) with 95% confidence intervals (95% CI) for hospital deaths due to each disease category are shown in Table 19 below, with comparison to similar estimates from the CUHK reports:
** p < 0.01; *** p < 0.001
Models with interactions between pollutants: The relative risks of hospital admissions for circulatory and/or respiratory disease in models with interaction terms between pollutants are as shown in Table 20 below:
NO2 interacted with SO2 for circulatory and/or respiratory diseases, with the estimated RR ranging from 1.11 to 1.13 at lower levels of SO2; and ranged from 1.05 to 1.07 at higher levels of SO2. RSP has an effect on circulatory and respiratory diseases combined at lower levels of O3 with RR 1.04 (95% CI: 1.02, 1.07) but no significant effect was seen at higher level of O3 . The effects for a pollutant were stronger at a lower level of the other pollutant interacting with it. The other pollutants for circulatory and respiratory diseases combined and separately were not significant. Models with interaction between pollutant and seasons: In studying whether the pollutant effects on hospital admissions for circulatory and respiratory diseases combined and separately, varied between seasons (i.e. spring: March - May; summer: June - August; autumn: September - November; winter: December - February), only the relative risk associated with O3 were found to differ in the spring, being higher than in the other seasons. The relative risk per 50 ug/m3 increase was 1.05 (95% CI 1.02, 1.08) in spring and 1.01 (0.99, 1.04) in other seasons for circulatory and respiratory diseases combined; and was 1.06 (1.03, 1.10) and 1.02 (0.99, 1.05) for respiratory disease alone. Effect of composite score of pollutants: A composite score of the concentrations of the four pollutants was derived (for method, see footnote for Table 21), which extracts the maximum correlation among the pollutants and explains 68% of the variations among them. Using this composite score as the independent variable instead of each of the pollutants, the results with all those significant health outcomes are shown in Table 21 below.
** p < 0.01; *** p < 0.001 # Composite score was generated from four pollutants (NO2, SO2, TEOM and O3) by principal components analysis and the first principal component was used which explained 68% of the variance with loadings of 0.491, 0.105, 0.726 and 0.469 respectively. (a) All ages (b) 0-14 years old (c) 15-64 years old (d) 65 years or older The results will be discussed in 5.2-5.4. Adequacy of 1995-96 models (hospital admissions): The residues for hospital admissions due to circulatory and respiratory diseases combined and separately, after fitting the model for each of the pollutants are shown in Figures 9(a) - 9(c). Patterns indicating unexplained variations in the models are observed. There were no discernible patterns in the residuals for circulatory admissions. There were some unexplained patterns in the residual plots for respiratory and respiratory and circulatory admissions combined. There may also be autocorrelations (i.e. correlation between consecutive observations) in the data. This was examined by the autocorrelation function plots in Figures 10(a) - 10(c). There was no evidence of autocorrelation in the circulatory admission residuals. However slight autocorrelation of less than 0.5 was present in the respiratory and respiratory and circulatory combined admission residuals. Predictive validity of 1995-96 models (hospital admissions): After obtaining the models from the 1995-96 data, the predicted number of hospital admissions due to circulatory and respiratory diseases combined and separately in the first half year of 1997 were obtained. The observed numbers in the first half year of 1997 and the predicted numbers with model using each of the pollutants are depicted in Figure 8 (a-c). Overall the models for prediction of circulatory and for prediction of circulatory and respiratory admissions were better (i.e. closer to the observed) than those for the prediction of respiratory admissions. In all the three health outcomes observed there were rising trends in the later part of the first half of 1997. These phenomena were particularly prominent in respiratory admissions indicating that the long term effects of other determinants of the disease might have changed. The models developed according to two yearsÕ data were not able to pick up the changes. But it would not invalidate the models to be used for estimation of air pollution effect on health. 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5.0 Discussion
| 5.1 | Validity and reliability of the models The validity and reliability of the models were ensured and assessed in the following ways:
|
||||||
| 5.2 | Summary of findings All relative risks referred to an increase of 50 ug/m3 in the concentration of the pollutant. Hospital admissions: Time trends, seasonality and weather conditions explained 31% to 79% of the variations in all the health outcomes under study. (In the CUHK report only those for admissions and hospital deaths due to circulatory and respiratory diseases combined were reported, which were 66% and 31% respectively.) Irrespective of the amount of variations unexplained after fitting the core model, all the pollutants under study showed significant effect on daily hospital admissions for circulatory and respiratory diseases combined and separately with relative risk (RR) estimates ranged from 1.03 to 1.10 in all ages overall. (The CUHK results were 1.03-1.18 in the RR estimates.) In analysis by age groups, apparently the effects of pollutants on circulatory diseases were stronger for the older age groups with significant RR of 1.05 to 1.10 found in 65 or older age group; and for respiratory diseases the effects by age groups were apparently j-shaped with RR 0.93-1.08 in the 0-14 years age group, 0.90-1.06 in the 15-64 age group, and 1.06-1.19 for the 65 or older age group. (In the CUHK report there were no comparable results.) For specific disease categories, NO2 , RSP and O3 were positively associated with admissions for asthma, RR 1.10-1.16; all pollutants were positively associated with chronic obstructive pulmonary diseases, RR 1.08-1.14; NO2 and RSP were significantly associated with ischaemic heart disease, RR 1.04-1.09. (In the CUHK results, only for asthma were available with RR (1.12-1.27.) For all ages and the 65 and older age group, NO2 and RSP exhibited the most consistent effects with estimates for RR 1.07-1.14 for NO2 and 1.03-1.10 for RSP, significant for all health outcomes; O3 exhibited the strongest effects with RR 1.04-1.19 all were significant except for ischaemic heart disease; SO2 exhibited the least effects with RR 0.91-1.12, all were significant except for asthma and ischaemic heart diseases. However as the pollutants are highly correlated, a composite score which summarises the four pollutants, was constructed and was found to provide the most consistent (i.e. closely estimated) for all the health outcomes under study with RR 1.06-1.11, all statistically significant except for asthma and ischaemic heart disease. (No corresponding results were from the CUHK study.) Hospital deaths: NO2 was positively associated with hospital deaths due to circulatory and respiratory diseases combined and separately with RR 1.10-1.14 (same RR 1.10-1.14 for CUHK results); and O3 was similarly associated with these health outcomes with RR 1.07-1.22 (CUHK 1.13-1.27). Similar to that for hospital admissions, the composite score for pollutant concentrations was also found to provide consistent estimates for effects of air pollution on hospital deaths with RR 1.05-1.09 (no corresponding results for CUHK). |
||||||
| 5.3 | Comparison with APHEA studies The estimates of relative risks in the validation study showed stronger and significant effects when compared with pooled results of Western European cities on hospital admissions due to respiratory diseases, asthma and chronic obstructive pulmonary diseases with our significant RR of 1.02-1.06 versus theirs of 1.01-1.03 for the 15-64 age group and 1.06-1.19 versus 1.02-1.04 for the 65 and older age group. Effects on hospital deaths were also more significant and higher in the HKU study than those in the pooled up estimates for total mortality from Western European studies with significant RR 1.10-1.15 versus 1.01-1.04 for circulatory diseases and RR 1.14-1.22 versus 1.04-1.05 for respiratory diseases. |
||||||
| 5.4 | Comparison with CUHK study However the estimates from the validation study, when compared with those from the CUHK study, were smaller with RR 1.04-1.07 versus 1.07-1.14 for hospital admissions due to circulatory and respiratory diseases combined for all ages; about the same for admissions due to circulatory diseases (RR 1.05-1.10 versus 1.04-1.12 for 65 or older); and smaller for respiratory disease (RR 1.06-1.19 versus 1.13-1.22 for the 65 or older age group). In the analysis for interaction effects between pollutants, the validation study followed the APHEA protocol in principle (i.e. defining the interaction term by multiplying a continuous pollutant concentration variable with the other pollutant dichotomized into high and low level in order to avoid multicollinearity10) and this is a more conservative approach. The CUHK study took a more aggressive approach (in that several co-pollutants with interaction terms were put in the same model and subject to model selection by means of a stepwise procedure). Relatively smaller numbers of interactions were found in the HKU study compared to the findings reported in the CUHK report. |
||||||
| 5.5 | Limitations of the Hong Kong study
Only two years of data: Unlike most studies in other places, the Hong Kong study only had two yearsÕ data for the analysis. It would not be realistic to confidently predict the health outcome in the third year of the study according to models developed in the previous two years, with reasonable accuracy. However the main objectives of this kind of studies were to assess the health effects of air pollution, but not to obtain prediction for health outcomes. Non-linearity of the effects: Although a moving average method was used to take account of the lag effect of air pollution, all the other covariates were modelled for their linear effects. Some other smoothing functions would be useful and have to be used such as the generalised additive modelling, for modelling the non-linear relationship between the health outcome and covariates. Refinement of models: There are discernible patterns in the residuals of some models. We have used different cycles (obtained from spectral analysis) in fitting the models and found that there were no changes in the fitness and estimates of the models. However plots of the residuals against pollution levels suggested that the unexplained variations are not related to air pollution. For the sake of uniformity we keep the same covariates of the original models for our results. Discrepancies between hospital deaths and total deaths: The hospital deaths included in this study represented only about half of all deaths occurred in the period. Also, they were analysed with the air pollutant concentration on the date of admission to hospital rather than on the actual date of death. These will produce discrepancies in the estimates compared to those when all deaths are included and analyses were based on date of death. New direction: There are new issues raised in the newly developed APHEA II16 protocol, such as in harvesting effects of air pollution on pre-mature deaths, and regional differences in the effect estimates. These issues were pointed out in a separate operation manual but were not addressed in this study. They should be taken into account in any future study. |
6.0 Conclusions
In this study we have examined the variations and covariations in daily concentrations of the four pollutants under study (objective 2.1). A composite score was derived from the four pollutants.
We have obtained, examined, cleaned and validated a series of hospital admission data (mostly from the Department? own resources) with health outcomes defined in accordance with the guideline of the APHEA project (objective 2.2).
We found and quantified the health effects of air pollution, representing by the daily concentrations of the four pollutants individually and compositely (objective 2.3). The estimates are consistent to but slightly greater than those from the APHEA studies.
We validated with the models developed in the earlier CUHK study by comparison with models independently developed and using different data which are comparable to those of the APHEA protocol. We have also obtained and provided in an operation manual the major source programmes, for the use and maintenance of the models for the continuous study by the Environmental Protection Department (objective 2.4). The critique of the CUHK study was in the following section and the operation manual in a separate documents.
| 6.1 | Critique of CUHK study
|
References
- Seaton A, MacNee W, Donaldson K, Gooden D. Particulate air pollution and acute health effects. Lancet 1995; 345:176-8.
- Pope CA, Thun MJ, Namboodri MM, Dockery DD, Evans JS, Speizer FE, Health CW. Particulate air pollution as a predictor of mortality in a prospective study of US adults. American Journal of Respiratory and Critical Care Medicine 1995; 151:669-74.
- Anderson HR, de Leon AP, Bland JM, Bower JS, Strachan DP. Air pollution and daily mortality in London: 1987-92. British Medical Journal 1996; 312:665-9.
- Committee on the Medical Effects of Air Pollution, Non-Biological Particles and Health. Department of Health. London: HMSO, 1995.
- National Air Quality and Emissions Trends Report, 1996. EPA-454/R-97-013. US Environmental Protection Agency, Office of Air Quality Planning and Standards. Research Triangle Park. NC, January 1998.
- Hedley AJ, Peters J, Lam TH, Ong SG, Wong CM, Tam AYC, Betson CL, Liu J, Spiegelhalter DJ. Air pollution and respiratory health in primary school children in Hong Kong 1989-92. Report to Environmental Protection Department, Sep 1993, pp245 and Appendices.
- Peters J, Hedley AJ, Wong CM, Lam TH, Ong SG, Liu J, Spiegelhalter DJ. Effects of an ambient air pollution intervention and environmental tobacco smoke on children's respiratory health in Hong Kong. International Journal of Epidemiology 1996;25:821-828.
- Tam AYC, Wong CM, Lam TH, Ong SG, Peters J, Hedley AJ. Bronchial responsiveness in children exposed to atmospheric pollution in Hong Kong. Chest 1994; 106:1056-60.
- Wong CM, Lam TH, Peters J, Hedley AJ, Ong SG, Tam AYC, Liu J, Spiegelhalter DJ. Comparison between two districts of the effects of an air pollution intervention of bronchial responsiveness in primary school children in Hong Kong. J Epidemiol Community Health (in press).
- Katsouyanni K, Schwartz J, Spix C, Touloumi G, Zmirou D, Zanobetti A, Wojtyniak B, Vonk JM, Tobias A, Ponka A, Medina S, Bacharova L, Anderson HR. Short term effects of air pollution on health: a European approach using epidemiologic time series data. The APHEA protocol. J Epidemiol Community Health 1996; 50:S12-18.
- Wong TW, Ho KM, Lau TS, Neller A, Wong SL, Yu ITS. Final Report: A Study of Short-Term Effects of Ambient Air Pollution on Public Health. The Chinese University of Hong Kong, 1997.
- World Health Organization. International Classification of Diseases, 1975 Revision.
- Schwartz J. Air pollution and hospital admissions for cardiovascular disease in Tucson. Epidemiology 1997; 8:371-7.
- Bacharova L, Fandakova K, Bratinka J, Budinska M, Bachar J, Gudaba M. The association between air pollution and the daily number of deaths: findings from the Slovak Republic contribution to the APHEA project. J of Epidemiol Community Health 1996; 50 (Suppl 1):S19-21.
- Schouten JP, Vonk JM, Graaf de A. Short term effects of air pollution on emergency hospital admissions for respiratory disease: results of the APHEA project in two major cities in The Netherlands, 1977-89. J of Epidemiol Community Health 1996; 50 (Suppl 1): S22-9.
- APHEA-2. Short-term effects of air pollution on health: A European approach to multiply, dose-response assessment and evaluation of public health significance. Scientific and Technical Report Part I.
- Katsouyanni K, Touloumi G, Spix C, Schwartz J, Balducci F, Medina S, Rossi G, Wojtyniak B, Sunyer J, Bacharova L, Schouten JP, Ponka A, Anderson HR. Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from the APHEA project. BMJ 1997; 314:1658-63.
- Touloumi G, Katsouyanni K, Zmirou D, Schwartz J, Spix C, Ponce de Leon A, Tobias A, Quenel P, Rabczenko D, Bacharova L, Bisanti L, Vonk JM, Tobias A. Short-term effects of ambient oxidants exposure on mortality: a combined analysis within the APHEA project. American Journal of Epidemiology 1997; 146:177-85.
- Zmirou D, Schwartz J, Saez M, Zanobetti A, Wojtyniak B, Touloumi G, Spix C, Ponce de Leon A, Le Moullec Y, Bacharova L, Schouten JP, Ponka A, Katsouyanni K. Time-series analysis of air pollution and cause-specific mortality: a quantitative summary in Europe (APHEA study) (unpublished).
- Spix C, Anderson HR, Schwartz J, Vigotti MA, Le Tertre A, Vonk JM, Touloumi G, Balducci F, Piekarski T, Bacharova L, Tobias A, Ponka A, Katsouyanni K. Short-term of air pollution on hospital admissions of respiratory diseases in Europe. A quantitative summary of APHEA study results. Archives of Environmental Health 1998; 53:54-64.
- Anderson HR, Spix C, Medina S, Schouten J, Castellsague J, Rossi G, Zmirou D, Touloumi G, Wojtyniak B, Ponka A, Bacharova L, Schwartz J, Katsouyanni K. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J 1997; 10:1064-71.
- Sunyer J, Spix C, Quenel P, Ponce de Leon A, Ponka A, Barumandzadeh T, Touloumi G, Bacharova L, Wojtyniak B, Vonk J, Bisanti L, Schwartz J, Katsouyanni K. Urban air pollution and emergency admissions for asthma in four European cities: The APHEA project. Thorax 1997; 52:760-5.
- Schwartz J, Spix C, Touloumi G, Bacharova L, Barumanmdzadeh T, Le Tertre A, Piekarksi T, Ponce de Leon A, Ponka A, Rossi G, Saez M, Schouten JP. Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. Journal of Epidemiology and Community Health 1966; 50 (Suppl 1):S3-11.